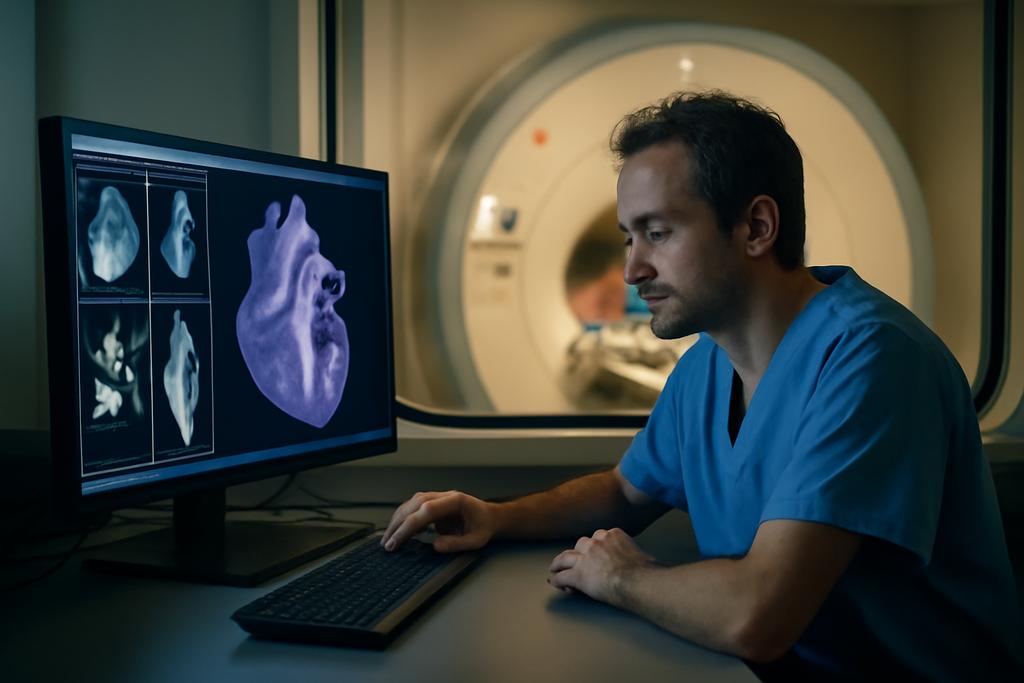
Cardiac MRI has become the quiet translator between a beating heart and medical insight. In pediatric and congenital heart disease, clinicians have long relied on 2D breath-hold cine scans to gauge how the heart fills and pumps. Yet those thin slices tell only part of the story, and asking a child to hold still for multiple scans can feel like chasing pixels in a moving target. A fuller picture — a true 3D view of the heart and vessels — could transform planning, diagnosis, and follow up. But practical 3D whole-heart imaging has trade offs in scan time, data handling, and the sometimes steep need for contrast. The result is a frustrating blend of clarity and complexity, especially when speed is of the essence in a busy clinic.
The team behind this new approach hails from the UCL Centre for Translational Cardiovascular Imaging at University College London and Great Ormond Street Hospital in London. Led by Vivek Muthurangu and Jennifer Steeden, with colleagues including Mark Wrobela and Michele Pascale, they built a pipeline that starts with a stack of real-time 2D cines captured during free breathing and then uses four deep-learning models to turn that bundle into a single, isotropic 3D cine that is fully segmented. In other words, they teach a computer to turn a rapid, breathless snapshot into a neatly organized 3D heart movie, ready for measurements and comparisons without extra fuss.
On screen, the result reads as a crisp 3D heart movie whose time has come in the clinic. It promises not just speed but a form of clarity that makes it feasible to quantify chamber volumes and vessel diameters quickly, with automatic segmentation that could plug directly into reporting. The study demonstrates that such a workflow can deliver data in well under a minute from scan start, a leap that matters when every minute in a busy pediatric suite counts. And because the models were trained on open data, the work is poised to ripple beyond a single hospital, inviting others to reproduce and build on the approach.
From Breath-Hold to Free-Breathing 3D Cine
Traditionally, cardiovascular magnetic resonance relies on breath-hold, 2D cines acquired in short-axis slices to size the ventricles and track function. A static 3D whole-heart scan can capture anatomy in one go, but its blood pool contrast is often weaker than the 2D cine, making it harder to delineate the chambers. To boost contrast, some centers rely on gadolinium-based agents, which adds cost and a layer of risk. An appealing shortcut is to concatenate a stack of breath-hold 2D cines along the slice direction, creating a pseudo 3D dataset. But this approach invites a host of problems: multiple breath-holds stretch the exam; slice thickness reduces resolution in the through-plane direction; misalignments between slices blur the anatomy; and bands of intensity differences can appear where slices join, dulling the image quality.
The real innovation here is to flip the problem: instead of fighting with slower, breath-held 3D methods or risky contrast, the researchers embrace real-time imaging. They acquire a sagittal stack of 2D real-time cines that can be captured during free breathing in under a minute, then apply a sequence of deep-learning reconstructions to turn those rapid, high-contrast slices into a single isotropic 3D cine. In short, they combine the speed of real-time imaging with the spatial uniformity of a true 3D dataset, then hand it to a set of models that clean up motion, harmonize contrast, sharpen resolution, and finally segment the anatomy automatically.
The practical promise goes beyond a single flashy trick. The team trained four distinct deep-learning models on open-source data to execute this pipeline: first they remove inter-slice contrast differences (de-banding), then correct slice misalignment caused by breathing (respiratory motion correction), next boost the slice-direction resolution (super-resolution), and finally label the heart chambers and major vessels (segmentation). The result is a fully segmented 3D cine dataset that unfolds across time, offering a dynamic, three-dimensional view of the heart at every moment of the cardiac cycle. It is a feat that felt almost science-fiction a few years ago, but here it is demonstrated in a clinical setting with real patients.
The Four DL Moves That Build a 3D Heart
The first module tackles banding. Real-time stacks learned to exhibit subtle intensity differences from slice to slice, which can undermine the blood pool contrast essential for clean segmentation. A conventional 3D UNet is trained to map a LowResresp+band volume to a LowResresp volume, smoothing away those inter-slice disparities. The loss function blends mean absolute error with gradient MAE, a choice that favors both pixel-level accuracy and smooth transitions across slices. The result is a harmonized stack that is ready for the next step without sacrificing the real-time information the technique depends on.
Next comes respiratory correction. Real breathing, even when free, nudges slices out of alignment. Here the team uses a 3D UNet with a twist: it preserves the slice dimension in encoding and decoding and yields separate x and y deformation maps for each slice. A non-trainable layer applies those maps to the input to produce a corrected volume. The goal is not to perfectly estimate the deformation fields themselves but to end up with an output volume that looks like it had been captured during a steady breath. The training blends MAE and GMAE losses with a regularization term on the gradient of the deformation fields to keep the corrections smooth and plausible across the entire stack.
After the motion is corrected, the system learns to super-resolve in the slice direction. The researchers employ an asymmetric 3D UNet that preserves the thin-slice information in the early layers and then ramps up isotropy in the deeper layers. The innovation here is to train end-to-end with respiratory correction, so the super-resolution model learns to tolerate any remaining misalignment and to recover true through-plane detail without simply interpolating from a low-resolution base. This joint training makes the final isotropic data more faithful to the original anatomy while keeping the computation practical for clinical use.
Last comes segmentation. A multi-class 3D UNet3+ predicts masks for the right and left atria and ventricles, the aorta, and the pulmonary arteries. The team uses a combination of Focal Tversky loss and a surface-area loss that emphasizes accuracy at boundaries, which are often the hardest places to delineate in imperfect data. The masks are then post-processed to remove stray islands and consolidate connected structures, yielding a clean, volumetric representation of the heart and great vessels across the cardiac cycle.
Training data for the enhancement and segmentation models came from open-source datasets — MM-WHS and HVSMR — and were standardized to 1.5 mm isotropic resolution with histogram equalization to stabilize contrast. The segmentation model was trained on a subset of those datasets, augmented with rotations and elastic distortions to mimic the natural variability seen in pediatric and congenital hearts. In all, the four models were trained separately and then deployed in sequence at inference time, frame by frame, to produce a fully segmented 3D cine for every moment of the heartbeat.
In terms of practical workflow, the study carefully reports the numbers. Acquisition of the stack of real-time 2D cines took about 42 ± 11 seconds in the prospective cohort. The offline de-banding, motion correction, and super-resolution steps required 7–9 seconds, depending on the number of slices. The segmentation step added another 20–25 seconds, culminating in a total offline reconstruction and post-processing time of less than 1 minute for each case. That is a striking contrast to conventional cine workflows, which can take many minutes to hours, especially for complete 3D assessments with full segmentation.
The authors also emphasize that the models were trained on openly available data and that the code is shared on a public GitHub repository. The broader implication is not just a single study with a clever trick but a blueprint for widespread adoption. If other centers can reproduce these results with their local data, it could democratize a new standard of rapid, automated 3D CMR that brings more patients into high-quality, quantitative imaging without adding cost or complexity to the protocol.
What It Could Change in Clinics
The prospective component of the study enrolled 10 participants spanning pediatric and adult ages with pediatric or congenital heart disease. The real-time multi-slice 2D stacks were acquired in roughly 42 seconds on average, markedly shorter than the breath-hold 2D cine stacks used as the reference standard. After the four DL steps, the final, automatically segmented 3D-cine was available in under two minutes from scan start, effectively delivering a complete 3D functional and anatomical dataset in a single pass. The speed is not merely a convenience; it has the potential to reshape how clinicians approach initial assessments, follow-ups, and planning for interventions in small children and busy clinics alike.
In terms of accuracy, the 3D-cine derived ventricular volumes and ejection fractions showed no significant biases relative to the conventional breath-hold 2D cine reference, with reasonable limits of agreement. Vessel diameters measured on the automatic 3D-cine data also aligned with the reference 3D whole-heart imaging, although there was a small but statistically significant overestimation in the right pulmonary artery diameter by about 0.8 mm. While not perfect, this level of agreement is promising for routine clinical decisions and, crucially, for rapid triage and monitoring in CHD patients where time and exposure to heavy protocols can be a real constraint.
A defining feature of the work is its emphasis on practicality. The researchers stress that their pipeline does not rely on contrast agents to achieve high blood pool contrast, instead leveraging the inherently high contrast of real-time cine, followed by intelligent processing. This could translate into safer, cheaper exams and more consistent workflows. And because the models are trained on open data and the code is openly shared, other centers can reproduce and iterate quickly, potentially accelerating a new standard across hospitals rather than confining it to a single lab.
Of course, the study is careful about its limits. The validation cohort is relatively small, and the per-volume processing approach means there is no explicit temporal coherence enforced across time points. In more complex congenital scenarios or rarer anatomies, extending the approach to a fully 4D consistency model could be warranted. The authors acknowledge these constraints while outlining a clear path forward: larger, multi-center trials, exploration of test-retest reliability, and continued refinement of the DL components to handle a broader range of anatomies. If those steps succeed, this method could become a mainstay in pediatric imaging, offering quick, automated, and interpretable 3D data without sacrificing diagnostic rigor.
Beyond the immediate clinical implications, the work is a cultural signal for medical imaging. It shows how a well-curated mix of real-time data, open-source training material, and modular DL tools can converge into a workflow that is not only faster but more accessible. The collaboration between University College London and Great Ormond Street Hospital, with authors spanning a wide range of expertise, demonstrates how rigorous clinical research can be married with practical software engineering to produce something that could be adopted in real-world clinics in a matter of months. And because the models operate on frames independently, the pipeline could be adapted to a variety of scanner setups, patient populations, and disease phenotypes, as long as there is a commitment to pushing data-driven improvements into the clinic.
In short, this study does not just propose a clever trick for 3D heart imaging. It sketches a future in which rapid, automated, high-contrast 3D CMR can be deployed in everyday care, with clinicians receiving fully segmented, time-resolved data at the speed of modern practice. The results are encouraging enough to warrant broader follow-up, and the authors’ commitment to open science lowers the barrier for other centers to join the experiment. If the next round of trials confirms these initial findings, the patient experience could improve dramatically: shorter scans, fewer recalls, faster diagnoses, and the ability to track heart growth and vessel development with a level of detail that previously required more invasive or time-consuming protocols.