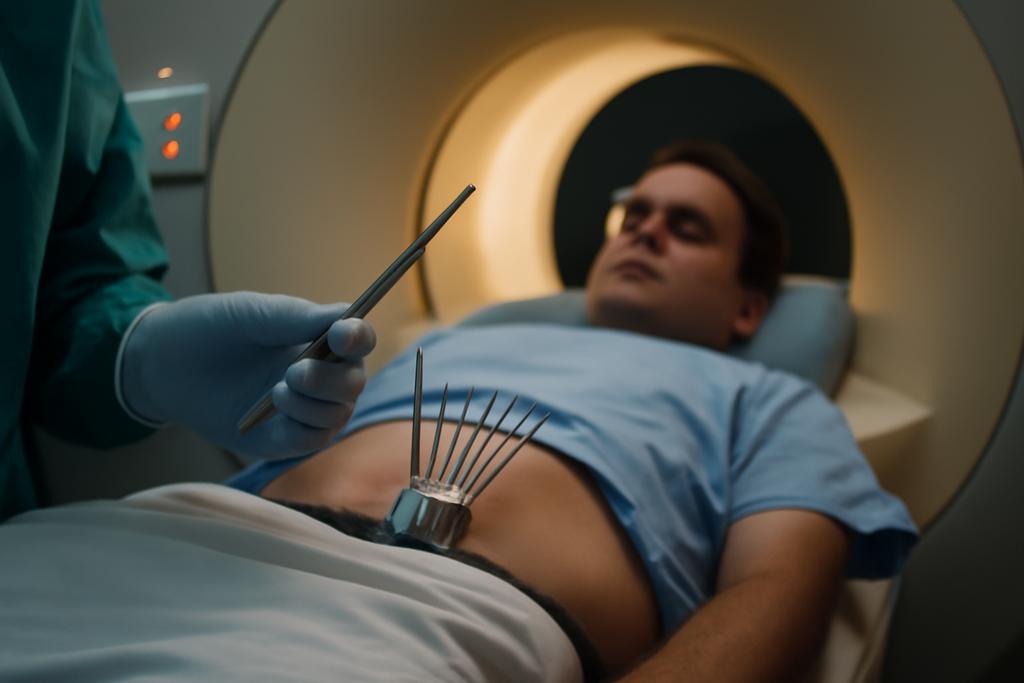
Imagine a microscopic dance, a precise ballet of radiation, targeting a tumor with the grace of a surgeon’s scalpel. This is the essence of brachytherapy, a cancer treatment where radioactive sources are carefully placed near tumors. While incredibly effective, planning this procedure is incredibly challenging and time-consuming –– until now. Researchers at Leiden University Medical Center, Amsterdam University Medical Centers, and Centrum Wiskunde & Informatica have developed a new AI-powered method that promises to revolutionize the process, and it all starts with fixing the tiny mistakes.
The Needle in the MRI Haystack
Brachytherapy for prostate cancer typically involves inserting multiple needles into the prostate gland. These needles deliver precisely targeted doses of radiation to the cancerous tissue. However, precise placement is crucial. To achieve this, doctors rely on medical images, typically MRI scans, to plan the precise location of each needle. Manually outlining these needles on the MRI images is incredibly tedious, prone to human error, and takes significant time away from patient care. Enter artificial intelligence.
Automatic needle reconstruction pipelines are already being explored. These pipelines generally work in two stages: first, an AI model (in this case, nnU-Net, a self-configuring deep learning model) segments the needles in the MRI, highlighting them from the surrounding tissue. Then, a post-processing stage takes the AI’s rough outline and refines it into a precise 3D model of each needle. The problem is that even the best AI models make tiny errors. These might be small gaps in the needle’s outline, stray points identified as needle material, or even a slight misalignment. These small errors, like misspellings in a sentence, can easily derail the final result. Existing post-processing methods, however, struggle to handle these subtle flaws.
Fixing the AI’s Mistakes: A Smarter Post-Processing System
The researchers tackled this problem head-on. Instead of trying to create a perfect AI segmentation model, they focused on building a post-processing stage that’s robust enough to deal with the AI’s inevitable imperfections. Their breakthrough involves several ingenious adaptations to existing post-processing techniques. Imagine an editor meticulously correcting a first draft: adding punctuation, merging fragmented sentences, removing extraneous words. That’s the essence of what they’ve accomplished. They developed methods that intelligently correct these errors, such as merging fragmented parts of the needle’s outline or removing erroneous points.
One key adaptation involves a new initialization step. In the previous methods, the system assumes needles all start in the same slice of the MRI. This isn’t always true, and the researchers devised a more flexible initialization strategy that works regardless of the needle’s starting point, making the system significantly more adaptable.
The researchers also introduced a clever “iterative removal” process. If the post-processing stage detects more needles than were actually inserted (a common consequence of the AI’s errors), it iteratively removes needles based on how much it improves the overall accuracy. It’s like carefully editing a text, deleting unnecessary words one by one until the meaning is clear.
The Results Speak for Themselves
The researchers tested their new method on a large dataset of MRI scans from prostate cancer patients at the Amsterdam University Medical Centers, comparing it to existing approaches. The results were striking. Their improved post-processing methods, particularly the one they call “MJung+”, reduced errors significantly and managed to get rid of false positives altogether. The median error in localizing the needle tips was an impressive 1.07 mm, and for the bottom of the needles it was just 0.43 mm. The median shaft error, a measure of overall accuracy, was 0.75 mm. Importantly, this was achieved with zero false positives––meaning they didn’t mistakenly identify any phantom needles. This level of precision is a major advancement.
The study, led by Tanja Alderliesten and Vangelis Kostoulas, highlights the potential of a two-pronged approach: improving both the AI segmentation and the post-processing stage. They showed that using a 3D segmentation model significantly reduced the number of errors compared to a 2D model, highlighting that refining the AI model itself is still important but that refining the post-processing stage can solve a large part of the issue.
Beyond Prostate Cancer: A Broader Impact
The implications extend far beyond prostate cancer brachytherapy. The techniques developed by Alderliesten, Kostoulas, and their team can be applied to other image-guided procedures involving needle placement. This could transform the planning process for a range of interventions, from minimally invasive surgeries to biopsies. The impact on patient care is profound; more efficient treatment planning means quicker treatment, less time under anesthesia, and improved patient outcomes.
The Future of Precision: Refining the Dance
While the results are incredibly promising, the researchers acknowledge the need for further validation. This next step involves having medical professionals evaluate the accuracy and usability of the new system in real-world clinical settings. However, the work is a significant step forward in the quest for more precise, efficient, and patient-centric healthcare. It’s a testament to the power of combining the best of AI with the ingenuity of human expertise to refine a process that has far-reaching implications.