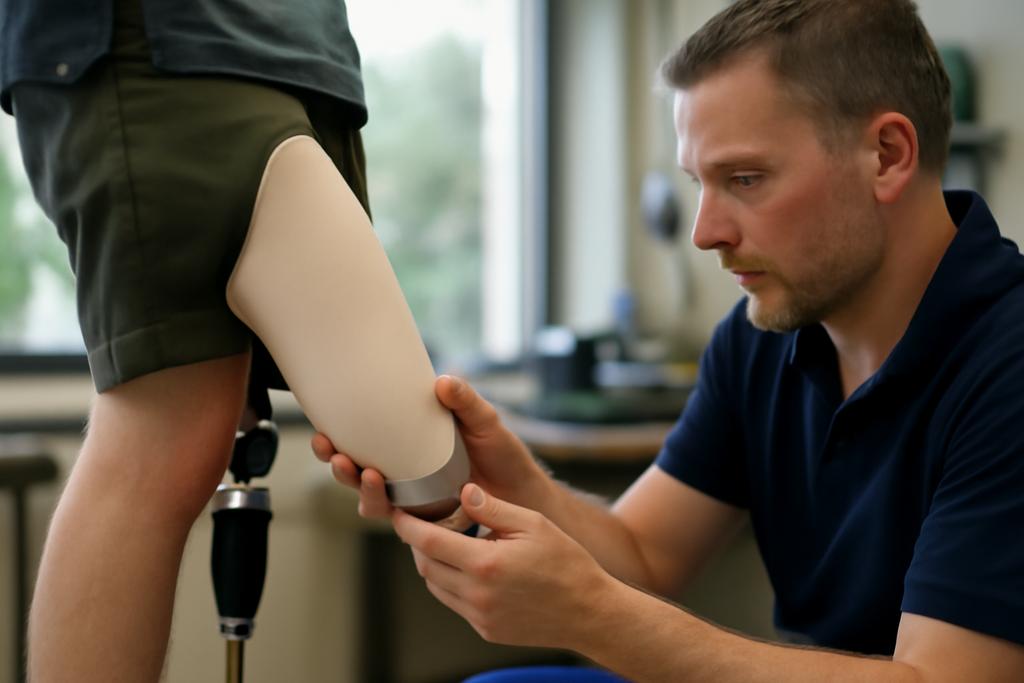
The socket is the intimate interface between a person’s residual limb and their prosthetic leg. It’s where comfort, control, and daily life hinge. And yet the way a socket is shaped is still largely a craft, perfected by clinicians who adjust plaster casts or digital scans with tactile intuition and years of experience. The new study from Radboud University Medical Centre in the Netherlands asks a bold question: could artificial intelligence help standardize this design in a way that preserves quality while scaling access, especially for millions who currently struggle to find a good fit?
Led by Celena Jordaan of the 3D Lab at Radboud University Medical Centre, the team gathered data from 118 people with transtibial amputations treated within the Dutch healthcare system. Each data point is two 3D worlds: a scan of the patient’s residual limb and a corresponding 3D model of the interior wall of the socket that the prosthetist designed. The goal isn’t to replace a clinician but to capture the prosthetist’s adaptations in a form a machine can learn from, and then re-create with consistency elsewhere. In places where access to skilled prosthetists is scarce—often in low- and middle-income countries (LMICs)—that kind of standardization could be a lifeline.
The World Health Organization has long underscored that access to assistive devices is uneven, with far too many people who could benefit from a prosthesis going without one. The researchers note that in LMICs only a sliver of amputees—about 5 percent—have access to prosthetic devices, and even when sockets exist, fit quality can be uneven. This study frames AI as a way to multiply expertise without replacing human judgment, a tool that could help clinics operate more consistently, more quickly, and at lower cost. The stakes aren’t just technical metrics; they’re about real people who need reliable mobility and comfort day in and day out.
From the start, the project was a careful balance of rigor and real-world practicality. The researchers pulled data from 3D scans of residual limbs and the surgeons or prosthetists’ own socket designs. They then pre-processed the data to tame the messiness that comes with 3D meshes: irregular point densities, different orientations, and how to compare two surfaces that aren’t perfectly identical. They used a morphable-model framework to standardize the meshes, ensuring the 3D data could be meaningfully compared across patients and practitioners. This kind of standardization is essential if an AI system is going to learn from a diverse set of real-world designs rather than from a perfectly curated synthetic dataset.
Crucially, the study didn’t just aim to predict a final socket shape. It asked whether the AI could learn to predict the adaptations a prosthetist would typically apply to move from a residual-limb scan to a usable socket. That distinction—predicting the adjustments rather than the absolute final shape—turned out to be a practical advantage, as the team discovered as their models learned from human expertise embedded in the data. The approach echoes a broader insight in AI for hands-on professions: sometimes the domain knowledge encoded in expert adjustments is more learnable than trying to guess an expert’s final product from scratch.
A new approach to socket design
To test their ideas, the team explored three families of AI methods, each trained in two related ways: (i) predicting the final socket shape directly from the 3D scan, or (ii) predicting the adaptations the prosthetist would apply to reach that socket shape. The three methods were a 3D neural network based on PointNet++ layers, a traditional feedforward neural network, and a random forest regressor. They ran the experiments with and without dimensionality reduction (PCA) of the 3D data to see whether a simplified representation might help or hinder learning.
The 3D neural network was designed to learn directly from the mesh, handling the irregularities of point clouds and meshes that don’t sit neatly on a grid—something traditional CNNs struggle with. The feedforward network offered a more conventional path, taking either the raw vertex coordinates or a PCA-reduced version as input. The random forest, a robust ensemble method, provided a different lens on the problem, especially when the data were reduced to their most informative components with PCA. Across all three methods, the common aim was the same: translate a 3D stump scan into a socket design that feels like it was crafted by a skilled prosthetist, but with the speed and replicability of a software process.
Before diving into results, it’s worth noting the data handling that made this work possible. The scans and socket models were aligned and standardized with a morphable-model pipeline (MeshMonk) to ensure uniform sampling of the surface geometry. Each processed example contained 3361 vertices connected to 6672 faces, a surprisingly manageable size for modern AI while still rich enough to capture the essential geometry of the residual limb. The team also explored a PCA-based representation not only of the stump and socket shapes but of the prosthetist’s adaptations themselves, enabling a dimensionality-reduced view of the same design problem.
Adaptations beat final shapes in prediction
The most striking takeaway from the results is where AI shined: predicting the adaptations rather than the final socket shape. Across the three learned architectures, models trained to forecast the required adjustments performed better than those trained to predict the socket’s final form. The best performer among them was a random forest trained to predict the adaptations in the non-reduced (full 3D) data, achieving a median surface-to-surface distance of 1.24 millimeters on the test set, with a first quartile of 1.03 millimeters and a third quartile of 1.54 millimeters. In plain language, half of the AI-predicted sockets were within about a millimeter and a quarter of the range of the prosthetist’s design—a level of precision that begins to approach human-level consistency when you’re dealing with complex 3D tolerance around soft tissue and bone.
Even when the researchers used dimensionality reduction, the random forest remained competitive. The results suggested that the structured, salient information captured in the adaptations—the targeted tweaks prosthetists routinely make to pressure-bearing regions and relief areas—carried most of the predictive weight. In short, the human element—the “how” of shaping based on feel and experience—was the part that AI could most effectively emulate, at least with the amount of data available in this study.
Looking at the predictions across all methods, the researchers confirmed a nuanced pattern: learning to predict the adaptations yielded lower error than trying to predict the ultimate socket geometry directly. The intuition is intuitive in hindsight. The socket shape is a composite product of the residual limb geometry and a clinician’s interpretive gaze—where to press, where to relieve pressure, how to accommodate soft tissue, how to balance load. The adaptations encode that interpretive layer. AI that can match that layer is, in effect, proxying the prosthetist’s design intuition rather than trying to replicate a finished sculpture from raw data alone.
What this could mean for care today and tomorrow
The study’s setting—data drawn from the Dutch health system, with collaborations among the 3D Lab, trauma surgery, and rehabilitation departments at Radboud University Medical Centre—grounds the work in a real-world clinical pipeline. It’s not a sterile dataset built for a competition; it’s a snapshot of how prosthetic socket design actually happens in routine care. The authors also flag the potential for broader impact: with more data, more diverse prosthetists, and more socket types, AI could help standardize quality across clinics that differ in resources, training, and patient demographics. In LMICs, where access to prosthetic care is limited and ideal socket-fitting expertise is scarce, such standardization could lower costs, shorten waiting times, and increase the odds of a comfortable, functional fit for more people.
Of course, the authors are careful with their language. They emphasize that more data will be needed to generalize beyond the Dutch dataset and to account for variables not captured in their current data—socket type (PTB versus TSB), patient weight, time since amputation, and other contextual factors that shape socket design. They propose several concrete next steps: allowing prosthetists to place landmarks on the stump before scanning to reduce inter-observer variability, expanding the dataset to include more diverse socket types and patient profiles, and exploring multi-modal models that weave in non-3D data alongside geometry. Each of these steps would push the AI closer to a dependable tool that augments, rather than replaces, human expertise.
The researchers also acknowledge a subtle, technical truth: the models’ performance currently reflects the limited size and heterogeneity of the dataset. In a field where a handful of millimeters can separate comfort from pain, every incremental gain in predictive accuracy matters. The paper’s results are encouraging but not a final word. They are a proof of concept—the demonstration that adaptive design knowledge, captured from clinicians, can be distilled into an algorithmic form and used to guide future socket fabrication. With more data and richer features, that capability could become a regular part of prosthetic care, not an exception in a research lab.
From bench to bedside and beyond
Behind the technical details lies a simple but powerful narrative: humans have learned to shape the prosthetic socket through a blend of anatomy, pressure mapping, and tactile feedback. AI can imitate the patterns of that expertise, offering a scalable scaffold that keeps quality high as clinics grow and reach more patients. The Radboud team’s work demonstrates a practical route to standardization without erasing sensitivity to individual anatomy or clinician judgment. It’s a reminder that advanced technology can assist in the most human of tasks—crafting a device that enables mobility, independence, and a better daily life.
Within this study, the most visible protagonist is not the machine, but the clinician’s artistry captured in data: the adaptations. The random forest’s 1.24-millimeter median error is not a flawless finish line but a meaningful milestone on a path toward broader access. If followed by larger datasets, more comprehensive features, and continued collaboration between clinicians and engineers, AI-assisted prosthetic socket design could become a standard option in clinics worldwide, reducing wait times, costs, and regional disparities in care. And that is a future worth aiming for.
In the end, the Radboud researchers—grounded in the 3D Lab’s facilities at Radboud University Medical Centre in Nijmegen and supported by programs like StitPro and ZonMw—have given us a concrete glimpse of how AI could translate skilled prosthetist practice into scalable, dependable design. It’s not a revolution that erases human touch; it’s a refinement that preserves care while multiplying its reach. For millions who live with limb loss, that combination could translate into more comfortable sockets, steadier mobility, and a daily reminder that technology, when steered by thoughtful clinicians, can widen the circle of access without narrowing the human approach that makes it work.